Acids are substances that, when dissolved in water, ionize or dissociate to release hydrogen ions (H+). This process of ionization is what characterizes them. The strength of an acid is determined by the extent to which it dissociates in water to produce these hydrogen ions.
When an acid dissolves in water, it forms hydronium ions (H3O+) by donating hydrogen ions to water molecules. The general formula for this dissociation is:
HA (acid) + H2O (water) → H3O+ (hydronium ion) + A- (conjugate base)
Here, “A-” represents the conjugate base of the acid.
The concentration of hydrogen ions (H+) in a solution determines its pH level. Acids have pH values less than 7, with lower pH values indicating stronger acidity. The greater the concentration of hydrogen ions, the stronger the acid.
Some common examples of acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), nitric acid (HNO3), acetic acid (CH3COOH), and citric acid (found in citrus fruits).
What is the Primary Ion in Acids?
The properties and generation of hydrogen ions (H+) and hydronium ions (H3O+) is fundamental in comprehending the behavior of acids, their impact on pH levels, and their role in numerous chemical reactions. These ions’ reactivity and ability to define acidity make them essential components in chemistry and various scientific fields.
Identification of the ion
Properties of hydrogen ions (H+)
Hydrogen ions (H+) are bare protons without electrons, making them highly reactive. They are extremely small in size, allowing them to move swiftly through solutions.
Being positively charged, they attract other negatively charged particles, influencing various chemical reactions.
Role in defining acidity
Hydrogen ions determine the acidic nature of a substance. Acids are classified by their ability to release H+ ions when dissolved in water.
The concentration of H+ ions in a solution dictates its pH level, with higher concentrations indicating stronger acidity (lower pH).
Formation of hydrogen ions in acids
Dissociation of acids in water
Acids dissociate into ions when added to water, releasing H+ ions. For instance, in the case of hydrochloric acid (HCl), it dissociates into H+ and Cl- ions.
This dissociation process varies based on the strength of the acid. Strong acids dissociate almost completely, while weak acids only partially dissociate.
Generation of hydronium ions (H3O+)
Hydrogen ions released by acids in water do not exist freely but quickly react with water molecules. H+ ions combine with water molecules (H2O) to form hydronium ions (H3O+).
This hydronium ion formation is crucial as it represents the actual species responsible for acidic properties in aqueous solutions. The presence of hydronium ions indicates the acidic nature of the solution, allowing for various chemical reactions typical of acidic substances.
How Do Acids Impact pH and Conductivity?
The pH scale’s relationship with H+ concentration provides insights into the strength of an acid or base and how it impacts its chemical properties. Additionally, recognizing how acids interact with bases to form salts and their ability to conduct electricity in aqueous solutions are fundamental aspects of their behavior in various chemical processes and applications.
pH scale and acidity
Relationship between H+ concentration and pH
The pH scale measures the acidity or alkalinity of a solution, ranging from 0 (highly acidic) to 14 (highly alkaline or basic), with 7 being neutral. pH is a logarithmic scale, meaning each unit change represents a tenfold difference in the concentration of H+ ions.
Lower pH values indicate higher H+ ion concentration and stronger acidity. For instance, a pH of 3 has ten times more H+ ions than a pH of 4.
Behavior of acids
Reaction with bases to form salts
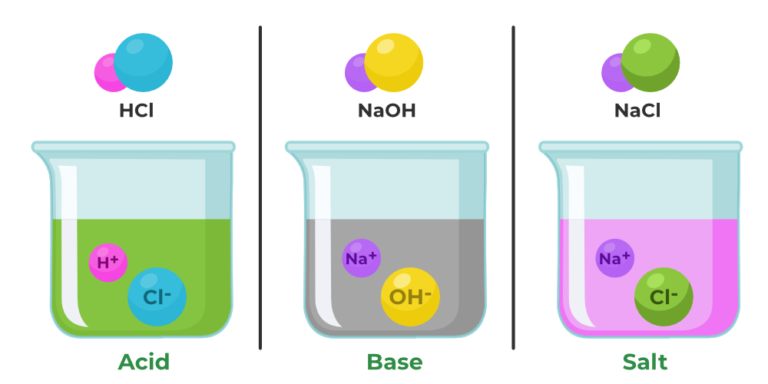
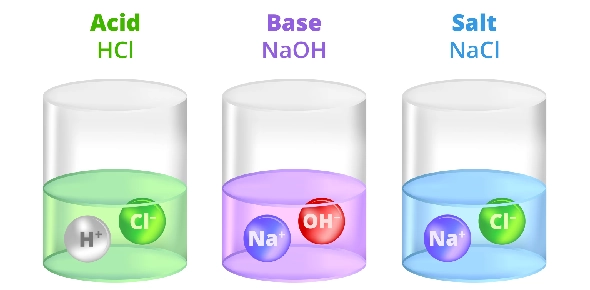
Acids react with bases in neutralization reactions, producing salts and water. In these reactions, H+ ions from the acid combine with OH- ions from the base to form water.
The remaining ions from the acid and base form a salt. For example:
Acid (HCl) + Base (NaOH) → Salt (NaCl) + Water (H2O)
Conductivity in aqueous solutions
Acids conduct electricity when dissolved in water due to the presence of ions. When acids dissolve, they release H+ ions and their corresponding anions into the solution, enabling the flow of electric current.
Strong acids, which completely dissociate into ions, exhibit higher conductivity compared to weak acids that partially dissociate.
What are Examples of Common Acids?

The characteristics, uses, and sources of these common acids, whether natural or synthesized, is essential for various industries, ranging from food production to chemical manufacturing. Moreover, recognizing the differences between strong and weak acids and their applications is crucial in both industrial and everyday contexts.
Common acids and their formulas
Hydrochloric acid (HCl)
- Formula: HCl
- Characteristics: A strong, highly corrosive acid found in the stomach’s gastric juices, aiding in digestion.
- Industrial Use: Widely used in chemical manufacturing, pharmaceuticals, and as a laboratory reagent.
Sulfuric acid (H2SO4)
- Formula: H2SO4
- Characteristics: Known as the “king of chemicals,” a highly corrosive and strong mineral acid used in various industrial processes, including battery manufacturing and fertilizer production.
- Industrial Use: Primarily used in the production of fertilizers, detergents, paints, and explosives.
Nitric acid (HNO3)
- Formula: HNO3
- Characteristics: A strong and highly reactive acid commonly used in the production of fertilizers, explosives, and as a laboratory reagent.
- Industrial Use: Essential in the manufacture of ammonium nitrate for fertilizers and in the production of various plastics.
Acetic acid (CH3COOH)
- Formula: CH3COOH
- Characteristics: Known as vinegar when diluted, it’s a weak organic acid responsible for the sour taste in vinegar. It’s also used in food preservation and as a solvent in the chemical industry.
- Industrial Use: Commonly used in the production of plastics, dyes, and pharmaceuticals.
Natural sources of acids
Citric acid in citrus fruits
Naturally found in citrus fruits like oranges, lemons, limes, and grapefruits. A weak organic acid provides the sour taste in these fruits. It is used extensively in the food and beverage industry as a flavor enhancer and preservative.
Oxalic acid in vegetables
Present in various vegetables like spinach, rhubarb, and beets. A dicarboxylic acid that, when consumed in large amounts, can interfere with calcium absorption in the body. However, in small quantities, it contributes to the tartness of these vegetables.